- One Mole Of A Substance Contains Atoms Or Molecules
- One Mole Of Carbon
- One Mole Of A Substance Contains How Many Atoms Or Molecules
- One Mole Of A Substance Contains Dash Atoms Or Molecules
- Mass Of One Mole
Converting Moles To Grams. One of the most common chemistry calculations is converting moles of a substance into grams. When you balance equations, you'll use the mole ratio between reactants and reagents. To do this conversion, all you need is a periodic table or another list of atomic masses. So, 1 mol contains 6.022×10 23 elementary entities of the substance. Chemical Computations with Avogadro’s Number and the Mole Avogadro’s number is fundamental to understanding both the makeup of molecules and their interactions and combinations.
››Convert mole to atom
Please enable Javascript to usethe unit converter.
Note you can turn off most ads here:
https://www.convertunits.com/contact/remove-some-ads.php
- The SI base unit for amount of substance is the mole. 1 mole is equal to 6.0221415E+23 molecule. Note that rounding errors may occur, so always check the results. Use this page to learn how to convert between moles and molecules.
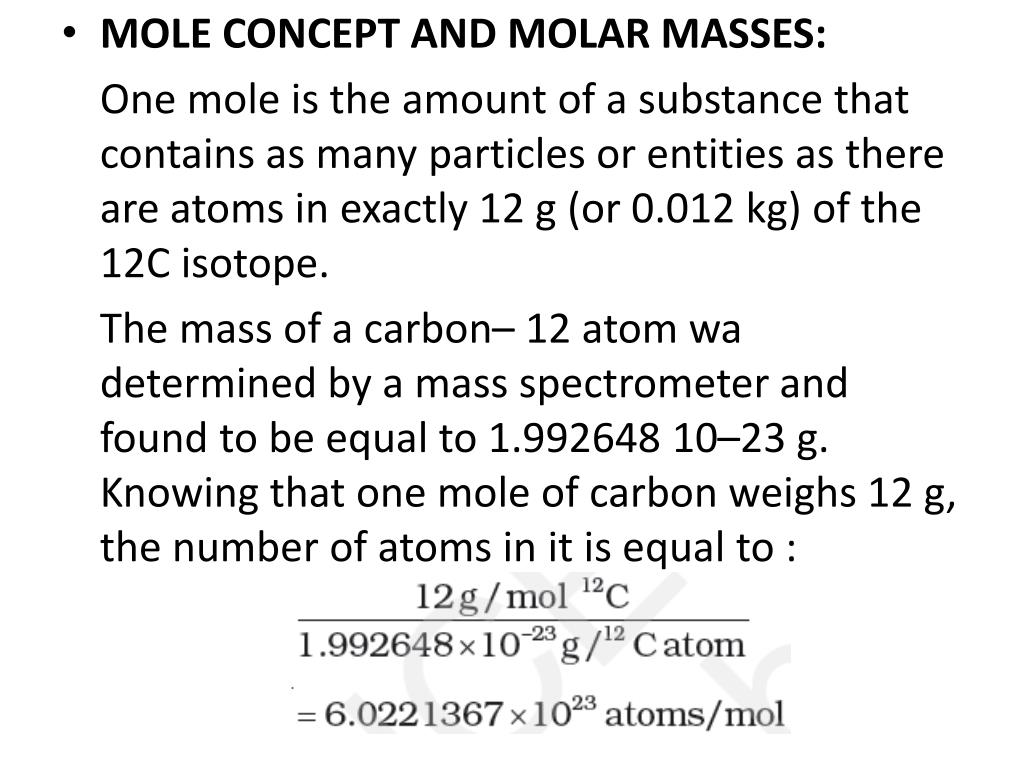
- A mole is the amount of a substance that contains as many particles as the number of atoms in 12 grams of what isotope? Chemistry Matter Isotopes.
››More information from the unit converter
How many mol in 1 atoms?The answer is 1.660538863127E-24.
We assume you are converting between mole and atom.
You can view more details on each measurement unit:
mol oratoms
The SI base unit for amount of substance is the mole.
1 mole is equal to 6.0221415E+23 atoms.
Note that rounding errors may occur, so always check the results.
Use this page to learn how to convert between moles and atoms.
Type in your own numbers in the form to convert the units!

››Quick conversion chart of mol to atoms
1 mol to atoms = 6.0221415E+23 atoms
2 mol to atoms = 1.2044283E+24 atoms
3 mol to atoms = 1.80664245E+24 atoms
4 mol to atoms = 2.4088566E+24 atoms
5 mol to atoms = 3.01107075E+24 atoms
6 mol to atoms = 3.6132849E+24 atoms
7 mol to atoms = 4.21549905E+24 atoms

8 mol to atoms = 4.8177132E+24 atoms
9 mol to atoms = 5.41992735E+24 atoms
10 mol to atoms = 6.0221415E+24 atoms

››Want other units?
You can do the reverse unit conversion fromatoms to mol, or enter any two units below:
››Common amount of substance conversions
mol to nanomol
mol to millimol
mol to centimol
mol to picomol
mol to decimol
mol to kilomol
mol to molecule
mol to micromol
››Definition: Mole
The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon 12; its symbol is 'mol.'

One Mole Of A Substance Contains Atoms Or Molecules
››Definition: Atom
One Mole Of Carbon
This site uses an exact value of 6.0221415 x 1023 for Avogadro's number. This is the number of atoms in 1 mole of a chemical element.
One Mole Of A Substance Contains How Many Atoms Or Molecules
One Mole Of A Substance Contains Dash Atoms Or Molecules
››Metric conversions and more
Mass Of One Mole
ConvertUnits.com provides an onlineconversion calculator for all types of measurement units.You can find metric conversion tables for SI units, as wellas English units, currency, and other data. Type in unitsymbols, abbreviations, or full names for units of length,area, mass, pressure, and other types. Examples include mm,inch, 100 kg, US fluid ounce, 6'3', 10 stone 4, cubic cm,metres squared, grams, moles, feet per second, and many more!
